Sistema
Praxis
PRODUCT NAME: MEDIIMPLANTES S.A. INTERNAL TUTOR FOR POSTERIOR THORACIC LUMBOSACRAL FIXATION
COMMERCIAL NAME: PRAXIS SYSTEM
REFERENCE: 102
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant Applications – UNS R56401, ASTM F136-02.
FUNCTIONAL DESCRIPTION
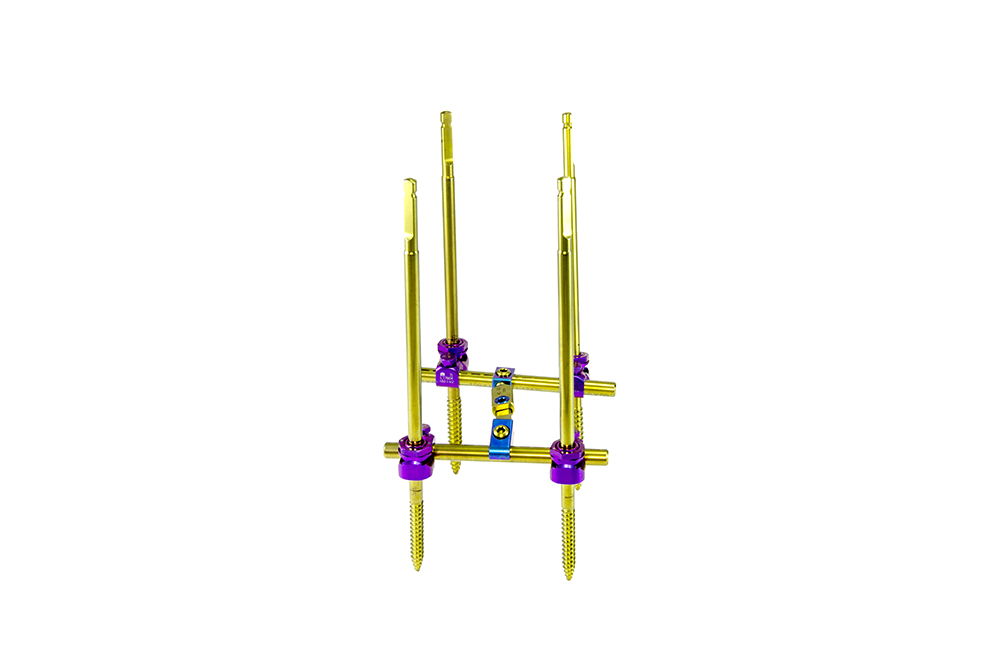
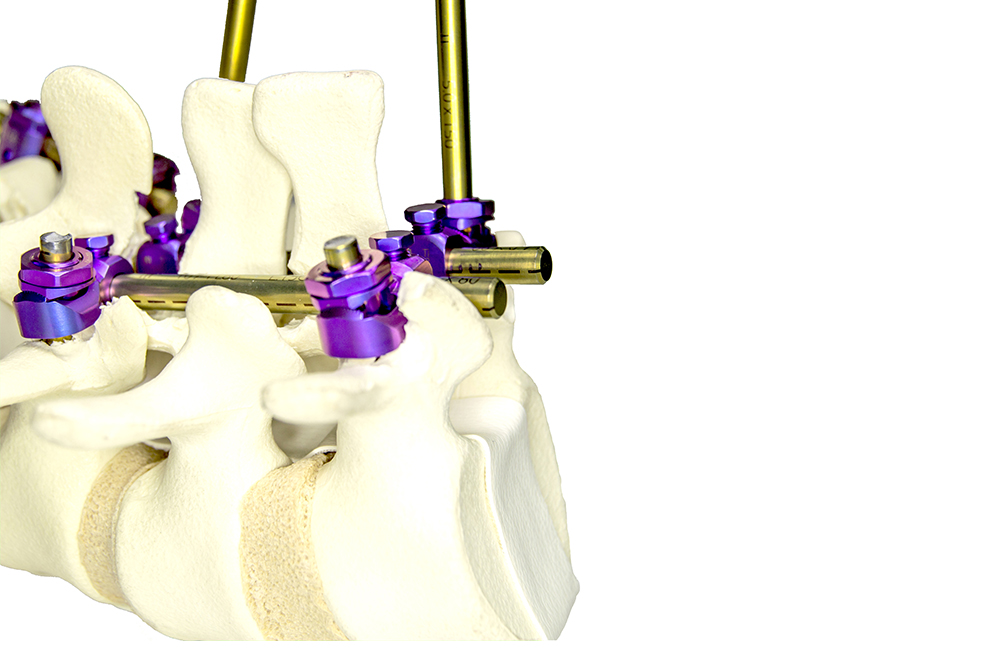
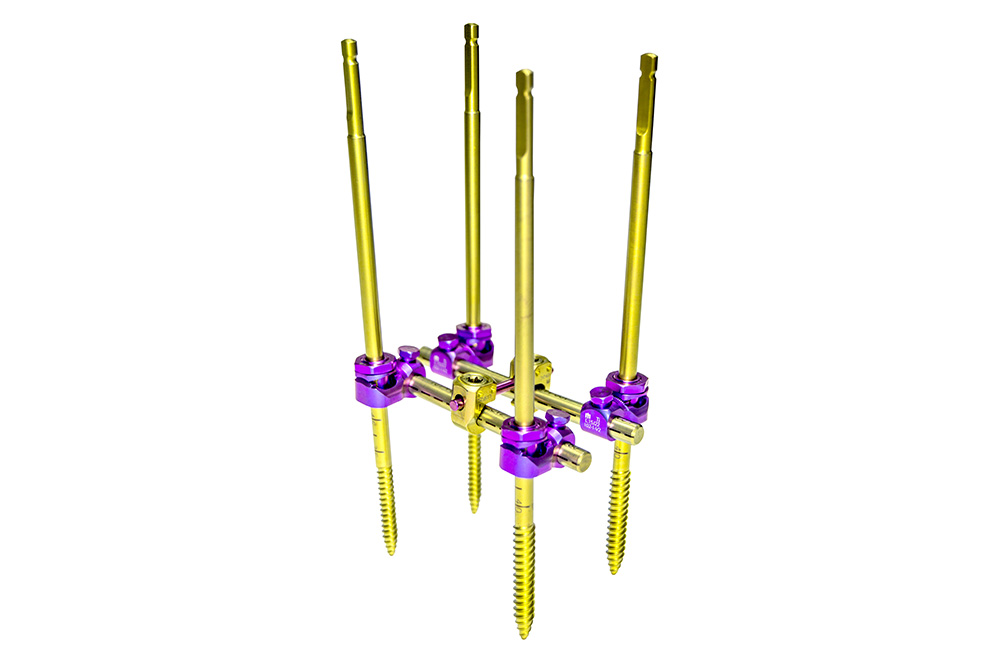
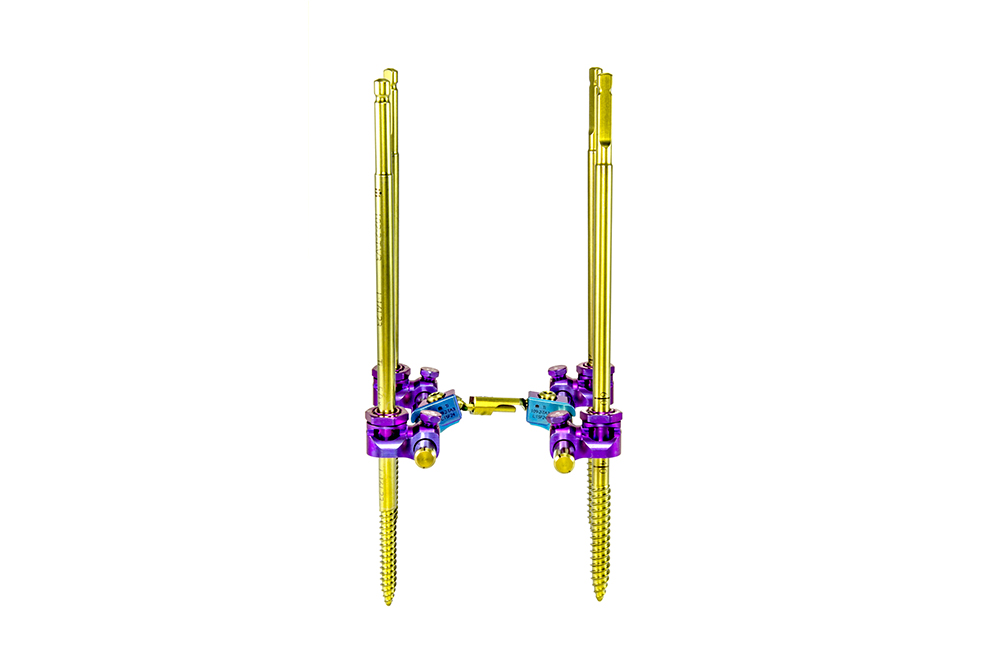
PRAXIS is a system composed of Schanz screws and ball joints and designed for use in the thoracic, lumbar, and sacral regions through posterior approach.
The ball joints allow to calibrate the angulation of the Schanz screws with ±15° in the sagittal plane, therefore allowing to correct spine anatomy in a very controlled fashion. The ball-joint’s design guarantees system rigidity after reduction and tightening.
INDICATIONS
– Ideal for fractures
– Posterior fixation of unstable fractures
– Tumors
– Degenerative spine instabilities
– Post-traumatic deformations
– Vertebral infections
– Spondylolisthesis
– Degenerative discopathies
– Scoliosis
– Thoracolumbar and lumbar kyphoscoliosis
STUDIES AND TESTS
– Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model- ASTM F 1717-04
– ASTM E8 Mechanical and metallurgical characterization.
The Schanz screws are easily inserted thanks to their self-threading thread profile and are available in different diameters according to the area to where they’re intended to be implanted (Ø5mm for thoracic region, Ø6mm for the lumbar, and Ø7mm for the sacral).
The system is completely compatible with the ADVANCED System for instrumentations requiring use of sub-laminar hooks or polyaxial screws.
CERTIFICATES
– INVIMA Sanitary Registration Nr. 2007DM-0000664
– ISO 9001 and ISO 13485 Registrations
PRESENTATION AND PACKAGING
Implants from the PRAXIS System are provided unsterilized, individually packaged, and individually laser-marked. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to display in an organized fashion for the surgeon’s use.
STORAGE AND AVAILABILITY
Immediate availability and easy storage. Does not need refrigeration nor any special handling procedures asides from maintaining cleanliness conditions to guarantee sterilization.