System
Advanced Pediatric System
PRODUCT NAME: TRANSPEDICULAR FIXATION SYSTEM
COMMERCIAL NAME: PEDIATRIC ADVANCED
REFERENCE: 14
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant Applications – UNS R56401, ASTM F136-02.
FUNCTIONAL DESCRIPTION
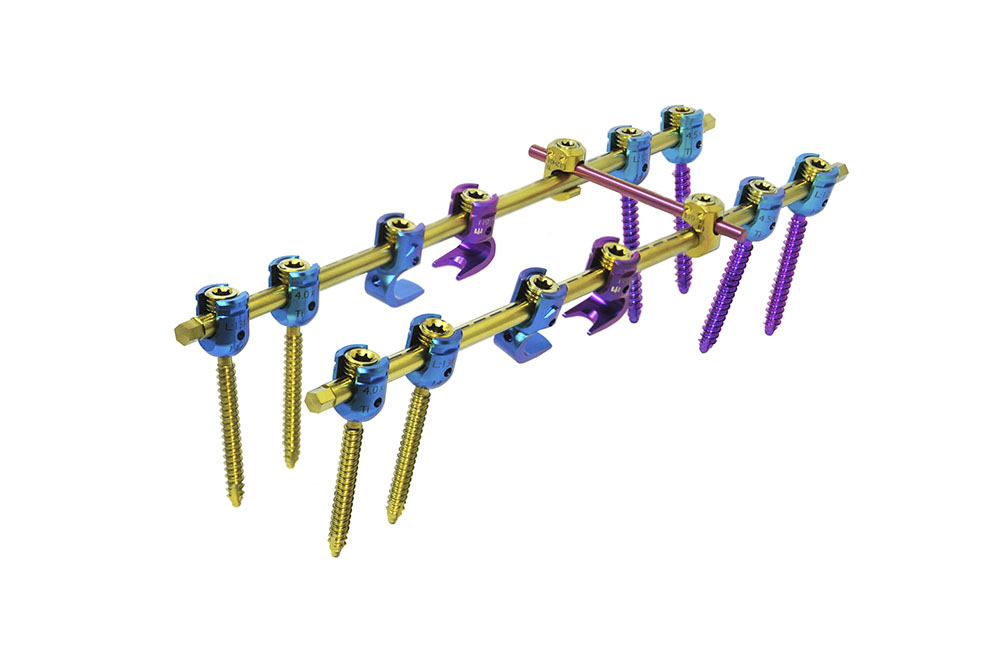
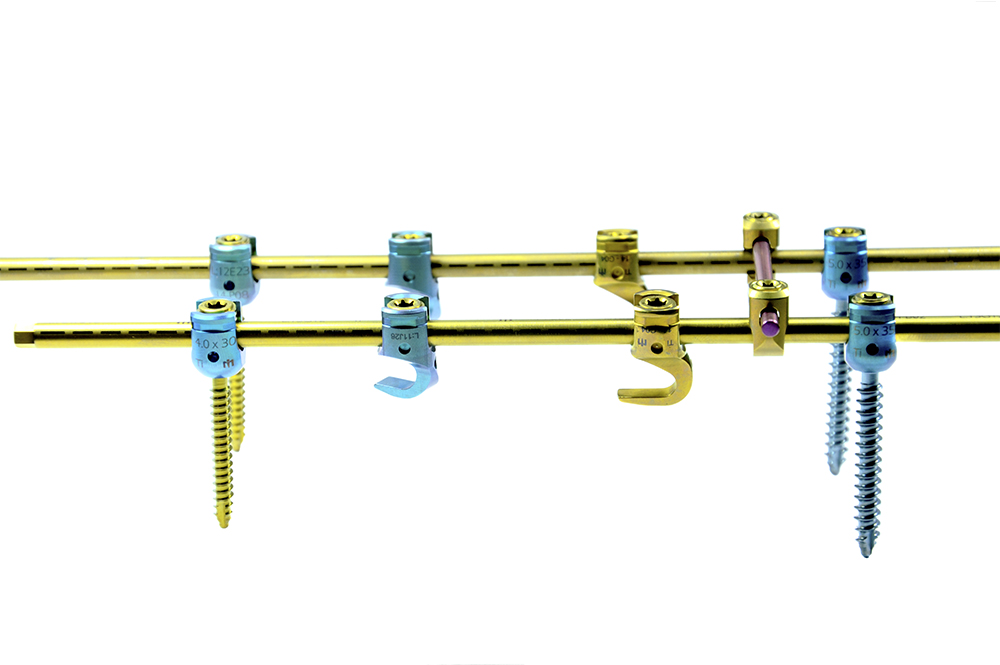
PEDIATRIC ADVANCED is a system composed by Monoaxial, Polyaxial, and Reduction Screws; Hooks, Reduction Hooks, Transverse Connector Hooks, and Longitudinal Bars. All of which are manufactured using a Titanium alloy (Ti6Al4V ELI, ASTM F136).
The system is designed to perform fixation through the pedicles to achieve immobilization and stabilization of spinal segments. It has implants of various sizes to adjust to the area of the spine to which they will be implanted.
The locking screw guarantees design-specific tightening force, either with the use of a torque limiter or through the screw’s mechanical fuse system.
INDICACIONES
-Malformations
-Tumors and infections
-Spondylarthrosis
-Thoracolumbar and lumbar scoliosis
-Thoracolumbar kyphoscoliosis
-Spondylolisthesis
-Posttraumatic and degenerative instability
-Degenerative discopathies
ESTUDIOS Y ENSAYOS
Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model- ASTM F1717-04
Caracterización Mecánica y metalúrgica ASTM E8.
CERTIFICADOS
Registro Sanitario INVIMA 2018DM-0000663-R1
Certificado ISO 9001 / Certificado ISO 13485
PRESENTATION AND PACKAGING
Implants from the PEDIATRIC ADVANCED System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks laid out in an orderly fashion for the medical specialist.
STORAGE AND AVAILABILITY
The implant must be stored and conserved in a dust-free, room temperature environment. It must be manipulated in accordance to applicable aseptic methods to avoid contamination.
Immediate availability.