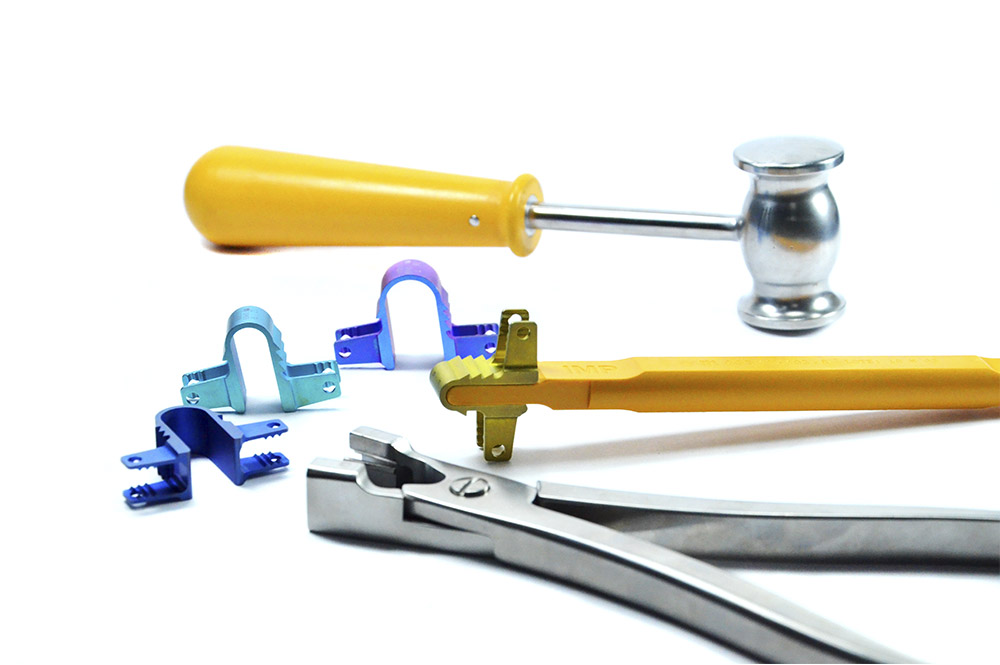
System
FLEXUS
PRODUCT NAME: DYNAMIC SPINE STABILIZATION SYSTEM AND ASSOCIATED INSTRUMENTS
COMMERCIAL NAME: FLEXUS SYSTEM
REFERENCE: 10A
MANUFACTURER: Mediimplantes SA.
FABRICATION MATERIAL: Ti 6Al 4V ELI (Extra-Low Interstitial)
FUNCTIONAL DESCRIPTION
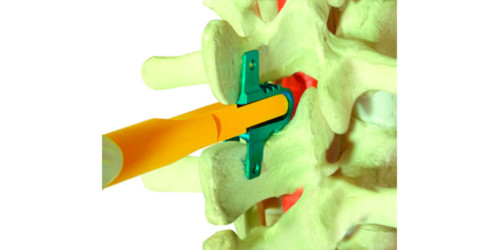
FLEXUS is a dynamic system used to stabilize the lumbar spine through posterior approach, indicated for pathologies associated with chronic pain. Posterior dynamic stabilization has the purpose of improving load distribution in joint structures in a way that stabilizes the segment but maintains movement capability. The system has implants in several sizes and longitudes that favor the lumbar spine’s various anatomical configurations.
INDICATIONS
– General Spine Instability
– Disc hernia in one or multiple levels
– Periodical Disc hernias
– Disc Degenerative Disease or iatrogenesis
– Lumbar Stenosis
STUDIES AND TESTS
– Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model –ASTM F 1717-04 Biocompatibility Test ISO 10993
CERTIFICATES
– ISO 9001 / ISO13485 Certification
– INVIMA Sanitary Registration Nr. INVIMA 2010DM-0006348
PRESENTATION AND PACKAGING
Implants from the FLEXUS System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to display in an organized fashion for the surgeon’s use.
STORAGE AND AVAILABILITY
Immediate availability and easy storage. Does not need refrigeration nor any special handling procedures asides from maintaining cleanliness conditions to guarantee sterilization.