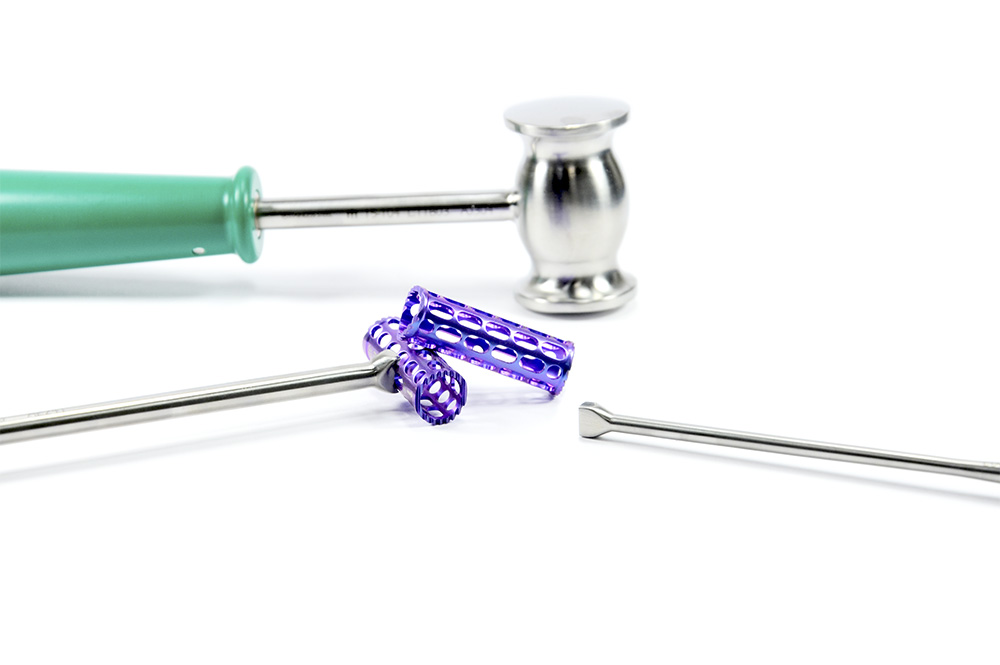
Sistema
CASTLE
PRODUCT NAME: CASTLE CERVICAL MESH CAGE SYSTEM AND RELATED INSTRUMENTS
COMMERCIAL NAME: CASTLE SYSTEM
REFERENCE: 15
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant Applications – UNS R56401, ASTM F136-02.
FUNCTIONAL DESCRIPTION:
CASTLE is a cervical mesh cage system of different sizes and angulation parameters between faces, indicated for patients with pathologies which require corrections through anterior approach cervical corpectomy.
The cervical mesh’s purpose is to maintain separation of bodies previously adjacent to the removed body and to provide anterior, central and posterior support of the cervical spine. It also maintains lordosis both immediately after surgery and for prolonged periods of time while bone fusion is achieved. It also works as a container for bone graft, used to achieve bone fusion; its frontal face reduces risk of bone graft migration to the spinal canal.
The jagged shape of its superior and inferior surfaces increases adherence to vertebral bodies and reduces risk of retropulsion. Furthermore, the increase in contact face diameter provides better support of the mesh and reduces subsidence.
INDICATIONS:
– Vertebral body replacement in patients with fractures or tumors, where anterior- approach partial or total corpectomy is performed.
– Tumors or fractures
– Cervical spine reconstruction
– Compressive myelopathy- associated cervical spine degenerative disease
TESTS:
– Tests Methods for Intervertebral Body Fusion Devices
– ASTM F2077-03
– ASTM E8 Mechanical and metallurgical characterization
CERTIFICATES:
– INVIMA Sanitary Registration 2011DM-0007142
– ISO 9001 and ISO 13485 Certifications
– Biocompatibility: ISO 10993-Cytotoxicity.
PRESENTATION AND PACKAGING:
Implants from the CASTLE System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks to arrange implant references in an organized fashion.