System
BLOCKED CERVICAL CELL
PRODUCT NAME: Blocked Intersomatic Cervical Cell
COMMERCIAL NAME: Blocked Cervical Cell
REFERENCE: CP
MANUFACTURER: NOVAX DMA S.A.
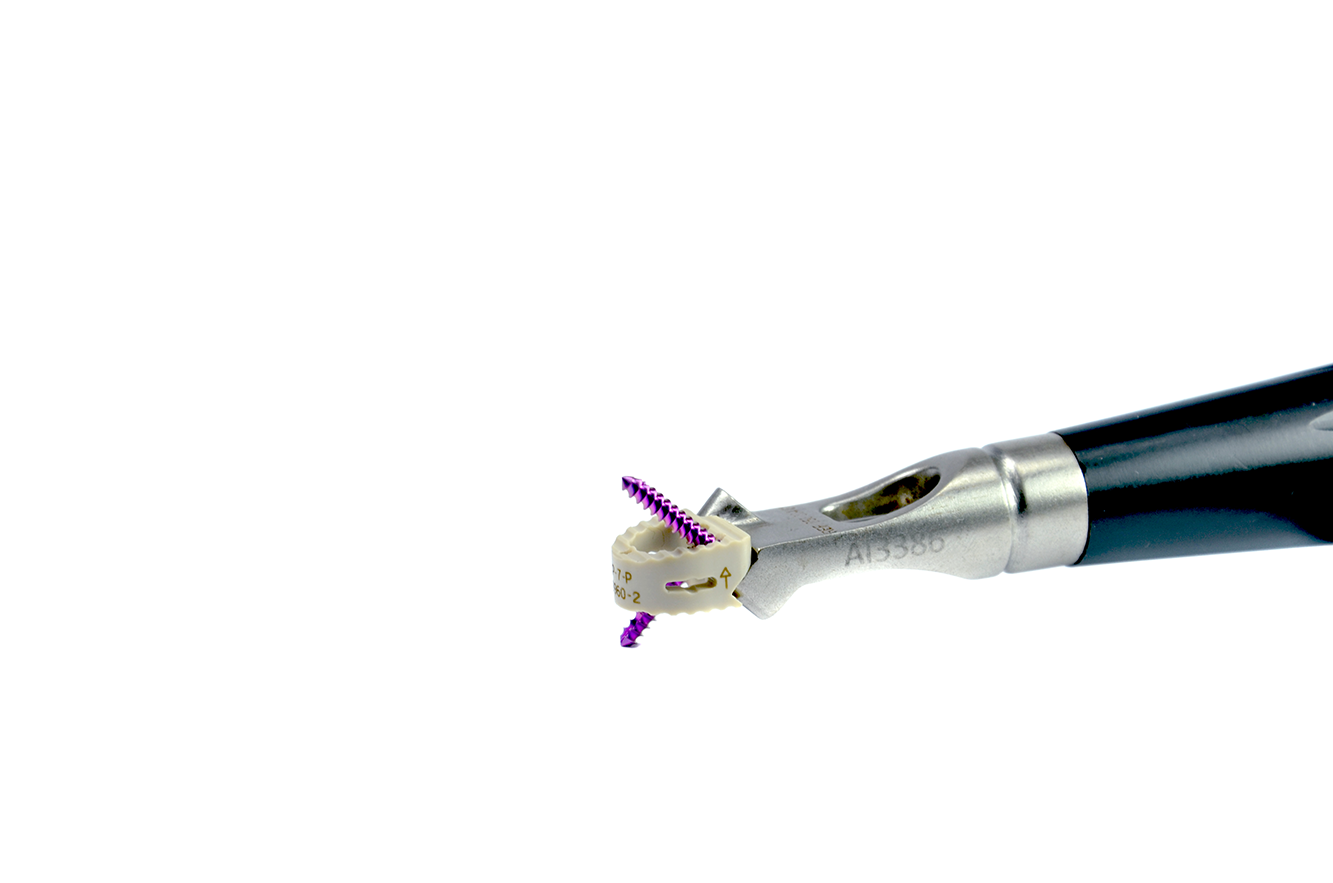
MANUFACTURING MATERIAL: The cells are manufactured using the polymer known as PEEK, according to ASTM F2026 norm specifications. The cells also contain grade 5 titanium (Ti6Al4V) markers (known as witnesses).
DESIGN AND INDICATIONS:
The main purpose of Intersomatic fusion cells is to correct mechanical deformation and restore the height of intervertebral disks, hence achieving stability of the affected segment until arthrodesis or intersomatic fusion are achieved.
The device’s dimensions are 12.4mm X 14mm, with variable heights from 5 to 8mm depending on the disk space needed to be replaced. Locking screws are available in 3 different length.
The cells are manufactured in PEEK, which is radiolucent. Therefore, they possess radiopaque titanium-alloy markers to identify their positioning during surgery and posterior monitoring.
The cells used for intersomatic cervical fusion are placed through an anterior approach and possess a central opening to allow for bone substitute insertion to promote arthrodesis.
This type of devices has a long record of reported applications and results in technical and scientific international literature. Cervical cells are a SIMPLE USE medical product and are NOT STERILIZED when supplied.
INDICATIONS:
Degenerative cervical discopathies (C3-C7)
Cervical Instability
Radicular foraminal compressions
Degenerative spondylolisthesis (degrees I and II).
Isthmic spondylolisthesis (degrees I and II).
Pseudarthrosis or failed arthrodesis.
The intersomatic cervical fusion cells can be used in combination with NOVAX DMA S.A.’s synthetic bio ceramic (PM 1621-63), either in cell dimension-specific packaging, blocks, or granules.
Important: This device is only intended for the cervical region. Once solid fusion is achieved, the device no longer serves any functional purpose.
REGISTRIES:
- INVIMA Sanitary Registry: 2009DM-0004960
PRODUCT CHARACTERISTICS:
- PEEK Material
- Straight
- Radiolucent
- Jagged transversal surface
- Radiopaque markers
- Space to allow for bone substitute insertion
- Heights: 6,7, and 8 mm
- Base dimensions: 12,4 x 14mm
- Probing implants for every height