System
ADVANCED
PRODUCT NAME: TRANSPEDICULAR FIXATION SYSTEM
COMMERCIAL NAME: ADVANCED
REFERENCE: 109
MANUFACTURER: Mediimplantes S.A.
MANUFACTURING MATERIAL: Ti 6Al 4V ELI (Extra low interstitial) Alloy for Surgical Implant.
Applications – UNS R56401, ASTM F136-02.
FUNCTIONAL DESCRIPTION
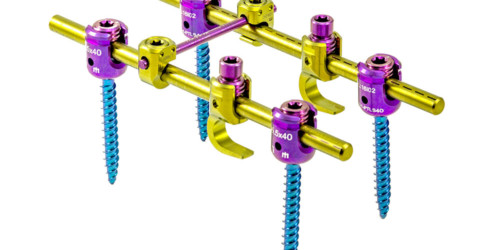
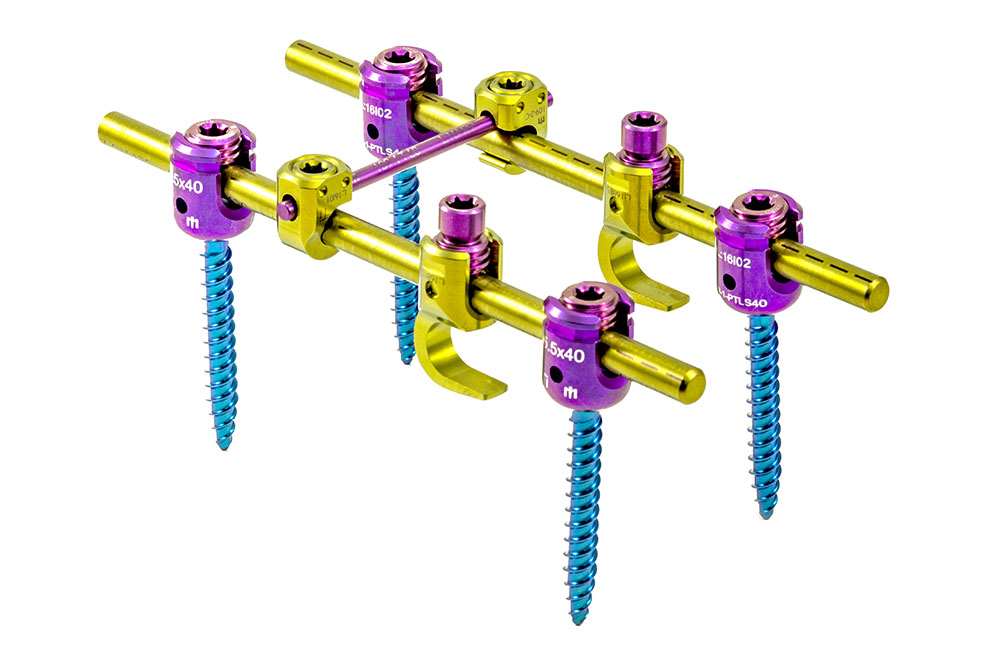
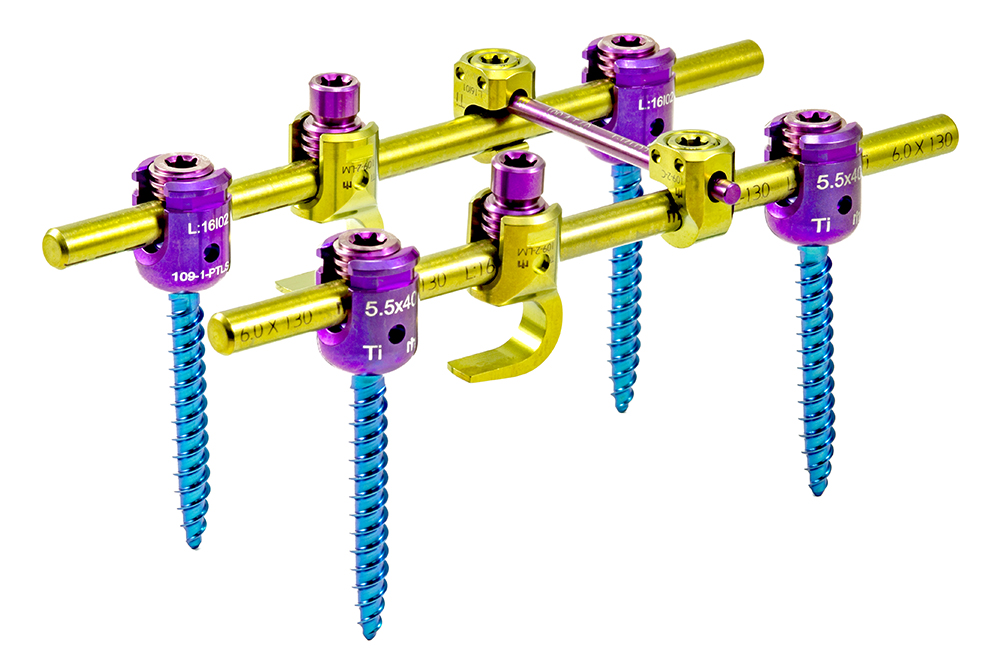
The ADVANCED System is designed to perform pedicle fixation to achieve spine segment immobilization and stabilization. It possesses implants of several sizes to adapt to the area of the spine to which they will be implanted.
ADVANCED is a system composed of monoaxial, polyaxial, bi-axial, and reduction-type screws, hooks, reduction hooks, transverse connector hooks, and longitudinal bars.
Polyaxial screws have a square thread profile that allows for a fastening force of up to 90 lb. x in. for increased stability. Appropriate locking screw fastening force is guaranteed either through the use of the torque limiter or through the screw’s mechanical fuse system.
The adjustable transverse connectors are designed to vary in length by sliding of their two bars (male and female) and allow a polyaxiality degree of +/- 20° in their hooks.
INDICATIONS
This is a spine implant system used for patients that present chronic and severe lower back pain caused by disc degenerative disease. Also used on patients with micro-instability.
Used for the treatment of pathologies such as:
– Malformations
– Tumors and infections
– Spondylarthrosis
– Thoracolumbar and lumbar scoliosis
– Thoracolumbar kyphoscoliosis
– Spondylolisthesis
– Posttraumatic and degenerative instability
– Degenerative discopathies
STUDIES AND TESTS:
– Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model- ASTM F1717-04
– ASTM E8 Mechanical and Metallurgical Characterization
CERTIFICATES:
– INVIMA Sanitary Registration 2018DM-0000663-R1
– ISO 9001 and ISO 13485 Certificates.
PRESENTATION AND PACKAGING
Implants from the ADVANCED System are provided unsterilized, individually packaged, and individually laser-marked. Each laser marking shows: product code, lot number, MEDIIMPLANTES logo, material symbol (Ti), and use-specific dimensions. Laser markings are permanent and allow for product traceability, even after implantation.
Primary Packaging: medical-grade paper bag for products to be sterilized and a laminated polyester film with a security band. It’s provided with non-toxic chemical indicators, fit for commercial sterilization methods, allowing for internal monitoring of sterilization parameters.
Secondary Packaging: Organizing racks that protect the implant from any possible mechanical damage caused by product movement to other locations.
Tertiary Packaging: Aluminum cases with safety mechanisms that contain all available organizing racks.
STORAGE AND AVAILABILITY
The implant must be stored and conserved in a dust-free, room temperature environment. It must be manipulated in accordance to applicable aseptic methods to avoid contamination. Immediate availability.